As we march into 2023, we wanted to take a moment to reflect on what has been a tremendously exciting past year for the Navi team.
Pictured: Navi founders (left to right) - Christiane Theda, Brad Bergmann, Shing Sheung, Mubin Yousuf, Wei Sue, Alex Newton
Whilst the current global environment has been filled with uncertainty, our team have been laser focused on developing the Neonav® ECG Tip Location System in order to deliver real impact in the treatment of critically-ill newborn and predicatric patients. This focus has delivered a year filled with significant progress and momentum, and has put our team in a strong position as we begin 2023 and beyond.
Below are some highlights of what has been an incredibly successful year for our team:
Built Up A Team Of Strategic Partners & Investors
This year has seen Navi receive a string of successful grant applications totalling over $4 million, which provides not only critical funding for upcoming product and clinical development work, but just as importantly provides strong validation from a wide-range of experts on the strength of our technology, team and vision. Below is a list of successful grant outcomes for 2022:
Clinical Translation and Commercialisation Medtech (CTCM) program
This $1.2m funding was granted to support the expansion of pilot study clinical activities, and the finalisation of device design and manufacturing, as well as execution of core commercialisation activities.
You can read the Australian Governments official announcement at https://www.health.gov.au/ministers/the-hon-mark-butler-mp/media/169-million-for-cutting-edge-medical-innovations
Accelerating COmmercialisation Grant (Tranche 2)
The Accelerating Commercialisation Grant is administered by the Australian Federal Government’s Department of Industry, Innovation and Science
The $600k in matched funding will support the completion of the next critical phase of product development work, as well as complete an upcoming interventional pilot study.
Medtech manufacturing capability program (mmcp)
Funded by the Victorian Government Department of Jobs, Precincts and Regions, the $178k MMCP funding aims to support local manufacturing of Victorian-based medtech companies in turning innovative ideas into real-life healthcare solutions.
Victorian Medical Research Acceleration Fund (vmraf)
The research project being funded will use Ultrasound technology to help further validate the accuracy of the Neonav® algorithm, as well as the use of ECG technology to accurately detect central venous catheter migration in critically-ill newborns.
The MMCP and VMRAF programs are administered by the Victorian Government’s department of Jobs, Precincts and Regions.
external capital investMENT
We have also had the support of some existing investors who have provided follow-on investment in 2022, as well as welcomed new investors to the Navi family, more exciting news on this coming soon!
Advancing our clinical research
The first phase of clinical studies came to a close in 2022, which provided our development team with valuable data that has helped advance the Neonav’s proprietary technology into the next generation prototype device.
The data collected involved recruiting over 130 newborn subjects across several clinical studies and procedures at the Royal Women’s Hospital NICU in Melbourne, Australia.
This work has laid the foundation for the next phase on clinical work, which includes the upcoming Ultrasound confirmation study, where we will be determining the accuracy of the Neonav® using Ultrasound technology, as well as an upcoming pilot study, which will be an interventional study using the final Neonav® product design.
Breakthrough Device Designation
The US Food and Drug Administration (FDA) has granted Breakthrough Device Designation for our medical device, the Neonav® ECG Tip Location System, as a method for navigating and positioning umbilical venous catheters in newborn patients.
The Breakthrough Device Designation is only given to devices that represent a significant breakthrough in effective treatment or diagnosis of life-threatening or debilitating conditions, and to technology where there is no approved or cleared alternative currently marketed in the US.
This Designation gives our team the opportunity to work closely with the FDA to develop the Neonav®, and will ultimately help expedite access for one of the most vulnerable and overlooked patient groups.
Watch the below video to learn more about the FDA Breakthrough Device Designation:
First Patent allowed in the USA
A significant milestone was achieved in 2022, with our first patent being allowed by the US Patent Office, which we expect to be granted in early 2023.
The patent (US 20210259778A1) titled “Catheter Location Determination In Pediatric Patients” covers a series of claims surrounding the placement of catheters utilising our proprietary ECG-based technology.
There is a significant amount of work underway in broadening the IP position of the Neonav®, as well as future technologies with broader clinical applications that will drive further value for the company and its shareholders.
Further expanded our US-based network
The Navi team attended the Association for Vascular Access annual conference in Minneapolis, MN, USA, where Navi's Chief Medical Officer A/Prof. Christiane Theda was selected to present and share Navi’s research findings on central line placement in critically-ill newborns.
The four day event is the premier meeting for vascular access healthcare professionals, providing the team an opportunity to explore industry insights, new product demonstrations, clinical advancements, and networking opportunities.

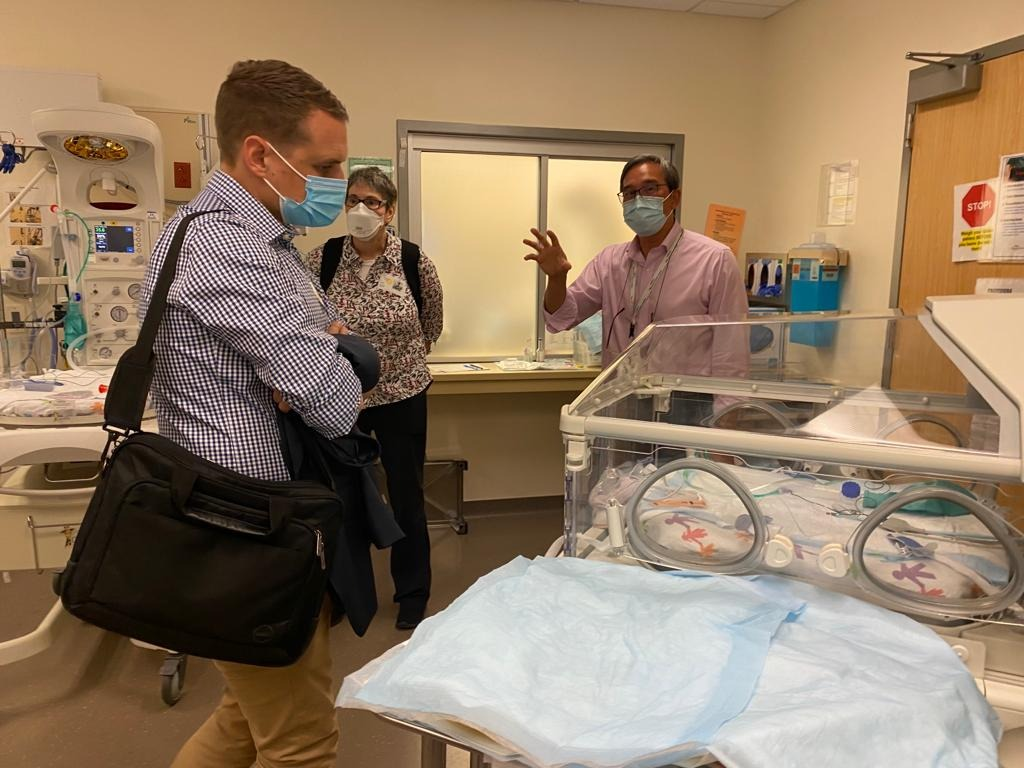



The team also spent time in San Francisco developing networks form the UCSF Benioff Children's Hospitals, where Navi Co-founder and COO Shing Yue Sheung won the Gold prize of USD$50,000 at the UCSF-Stanford Pediatric Device Consortium’s Michael R. Harrison Innovation Symposium pitch competition.
Pictured: Shing Yue Sheung being awarded the gold prize at the UCSF-Stanford PDC pitch competition
Advanced Product Development
The Neonav® product development showed significant progressed during the past year, which included:
Working with front line health care workers across Australia and the USA to gather valuable device feedback.
Worked with engineering partners to refine the concept direction for the Neonav® including industrial design, improved user interface, optimised hospital workflow integration, and finalise design decisions on the device ecosystem.
Upgraded our R&D workspace to help test and develop current and future innovations.
The above work will be implemented into the next generation prototypes that will be used in our upcoming pilot study in late 2023.
We Supported the Next Generation Of Innovators
Navi was proud to be the official major sponsor of the Melbourne University Biomedical Engineering Society (MUBES) 2022 Medithon, which was a multi-day event was designed to help graduate students apply their learnings towards solving real-world healthcare problems in the pediatric space, and pitch their very own prototype idea to a panel of clinicians, academics and industry professionals.



Navi CTO Mubin Yousuf delivered the keynote presentation, as alumni of the University of Melbourne, the founders of Navi are thrilled to be able to give back and support the next generation of medtech innovators.
Media Highlights
Navi co-founder and Chief Medical Officer A/Professor Christiane Theda was a guest on Seven Network's “House of Wellness”, where she spoke about our medical device, the Neonav® ECG Tip Location System.
The powerful segment captured the challenges that critically-ill newborns and their families face with a touching story from the Constable family, whose child Nash had spent several weeks in the Neonatal Intensive Care Unit and took part in our clinical research at The Royal Women's Hospital.
As always, it is a huge motivation to see the positive impact our technology can have on both newborn patients and their families, and it's these stories that drive the Navi team to innovate and grow.
Navi CEO Alexander Newton and Chief Medical Officer Christiane Theda were also guests on the "Beyond The Medicine Cabinet" podcast series with Zoë Callister-Hakewill.
The episode covered the story of Sarah and James, whose son Archie spent 210 days in the NICU, and discussions about how Navi's medical innovation, the Neonav® ECG Tip Location System, can improve the standard of care in newborn and pediatric patients
You can listen to the full episode below:
Continued Team Growth
The Navi team welcomed Katerina Barons as a full time Data Analyst, and Shehan Wisidagama as our new R&D engineer. Both Katerina and Shehan assist in critical value-add activities in the development of the Neonav® and other technologies.
Pictured: Katerina Barons - Data Analyst
Pictured: Shehan Wisidagama - R&D Engineer








